Biological Products:
Bioaugmentation products for Wastewater applications in Papermills, Refineries, Chemical, Tanneries, Municipalities, Textiles, Steel, Agriculture, Animal feedlot, Gun Powder plant, Food and Beverage- Dairy Products, Orange Juice factory, Wineries, Cookie factory, Vegetable processing plant, Meat packing, Barbecue Restaurant, Aquaculture, Ornamental Ponds with algae , CAFO, Nursing homes, Military, Campgrounds, Universities, Regulatory agencies, River and Lake remediation
Lab Services:
Filamentous Identification Lab Service. One reason to identify filaments is to determine the filaments characteristics and then determine the type present. If the type is found out, a root cause can usually be associated with a particular filament. If the cause is known, then a correction can be made to alleviate problems. Chlorination is only a quick fix. Without process changes, filaments will grow back after chlorination. Wastewater Biomass Analyses and Cooling Tower Analyses also available
Audits and Consulting:
At Environmental Leverage® Inc., we have a team of experienced individuals who come into your plant with a fresh pair of eyes. The system is checked from influent to effluent. System optimization, equipment efficiency and operational excellence are key components explored. Key Benefits Equipment efficiency Total Cost of Operation reductions Reliability and safety An onsite audit is conducted to examine system parameters, process controls, and current monitor and control procedures. A physical walk-through is conducted, process flow diagrams are examined, previous design criteria are examined and current standard operating procedures are evaluated along with data logs.
Training Materials:
Training is an integral part of any job. Not everyone is at the same level of training. Many people want beginning concepts and basics. Some need technical information or troubleshooting. Some want equipment, technology or process information. We have developed a full set of Basic training, Advanced training, Filamentous Identification the Easy Way as well as custom training CD's Manuals. We also provide hands-on training classes and soon will have an Online "E-University".
|
Industry Troubleshooting-Nitrification Math BalanceLatest News!
What's New!
We have just added "Virtual Audits" to our capabilities. Check out our new Services. We are in the process of developing new courses for our ""Online E-University" in order to meet the needs of our global customers that cannot travel to our public classes.Visit our new website www.WastewaterElearning.com/Elearning
Nitrification -Why a Total Nitrogen balance is important!Nitrogenous Compounds discharged from wastewater treatment plants can have many harmful effects.
Many municipalities and industrial facilities are required to remove ammonia from their final effluent. Some wastewater treatment plants are required to have a Total N permit, this includes ammonia as well as organic nitrogen, and nitrates. Nitrogen removal is achieved two ways. First the carbonaceous bacteria will use a small portion as a nutrient source. The typical ratio is 100 parts carbon to 5 parts Ammonia. Many times, though if a wastewater treatment plant has too many filamentous bacteria, this ratio can be quite lower. Some filamentous bacteria grow quite well in a nutrient deficient environment and do not consume nutrients quite the same as the floc forming bacteria. Try to make sure that your biomass has a higher portion of floc formers than filamentous bacteria.
The second way to remove ammonia is through nitrification. Many people do not understand that it is a completely different process and completely different species that perform nitrification. Carbonaceous bacteria use the carbon as a food source. Nitrosomonas and Nitrobacter use Carbonate as their food source and the ammonia is just their energy transfer source. They do not "eat" the ammonia or make it dissappear. Most of the NH3-N is used as an energy source. It is used in a non-assimilative way so only a small amount of biomass (sludge) is produced. Carbon dioxide (CO2) or carbonate is used as the carbon source in nitrification. That is why alkalinity is extremely critical in nitrification. 7.14 parts of alkalinity are required for each part of ammonia to be removed.
The Nitrification Process First Conversion (Ammonium to Nitrite) Nitrosomonas bacteria oxidize ammonium to nitrite via hydroxylamine.
Second Conversion (Nitrite to Nitrate)
Nitrification also occurs 3-4 times slower than carbonaceous oxidation. Upsets to a plant can take nitrifiers weeks to recover for nitrification as opposed to days or hours for carbon bacteria. For each 1-gram of NH3-N oxidized to NO3, 0.15 grams of new bacteria cells are formed. Sludge generation from nitrifiers is minimal in a wastewater treatment plant.
Oxygen levels are also critical for nitrification. Higher levels of oxygen are required for nitrification than for carbon removal. As ammonia is removed it is transformed. 4.5 parts of O2 are needed for every part of NH3 to be degraded.
Controlling Factors for Nitrification: Dissolved Oxygen Alkalinity (pH) Wastewater Temperature Nitrogenous Food Detention Time MCRT, F/M, or Sludge Age Toxic Materials
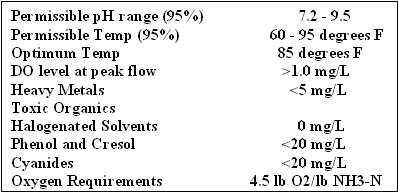
Dissolved Oxygen- Nitrification has a substantial oxygen requirement. 4.5 lb O2 is required per lb NH4+-N to be converted. Aeration Tank D.O. usually maintained 1.0 to 4.0 mg/L .
ph, Alkalinity Nitrification rates are rapidly depressed as the pH is reduced below 7.0. pH levels of 7.5 to 8.5 are considered optimal. Typical refineries run at a pH of 8-9 with no problems. 7.14 lbs of M-alkalinity are destroyed per lb of ammonia-nitrogen oxidized.
Nitrification is inhibited at lower wastewater temperatures in wastewater treatment plants. Up to five times as much detention time may be needed in the winter versus the summer months since the activity drops significantly. During winter months, increasing MLVSS, MCRT will help as well as lowering wasting. A desired range is 60° to 95° degrees F. Below 40 nitrification will probably not occur.
Nitrogenous Food BOD5/TKN Ratio -The fraction of nitrifying organisms decreases as this ratio increases. Many times influent TKN is not measured. Refineries and Chemical plants often have amines in the influent and are used to measuring this and accounting this in the total nitrogen balance. Municipals are not used to doing this, but probably should if they have any industrial pretreatment adding to their influent, or if they have a ton of food plants or restaurants adding high loading to their plant. Many times the new cleaning and sanitation compounds have quaternary amines and biocides as a component of the cleaning and sanitizing compounds. These can add a significant loading to the plant. Especially since it takes time to break down the amines first, before the ammonia is released and can be recognized or utilized by the nitrifiers.
Detention Time The time required for nitrification is directly proportional to the amount of nitrifiers present. Because the rate of oxidation of ammonia is essentially linear, short-circuiting must be prevented. Minimum detention time is approx. 4 hours at 22 ° to 24°C. Many plants run very old sludge ages. Be careful of this. A sludge age that is too old will have problems with sudden changes in the influent and will have a higher problem with toxicity issues than a medium to old sludge. Let as much of the ammonia removal happen by the carbonaceous bacteria first, and use your nitrifiers as a final polishing only.
MCRT, F/M, or Sludge Age When reviewing the performance of your activated sludge process for the selection of an optimum F/M ratio, MCRT, oxygen requirements, etc. , the requirements of the nitrification microorganisms must be taken into account in addition to that of the heterotrophic (carbon degrading) bacteria.
Inhibition to nitrification by Toxic compounds Many compounds can be toxic to nitrifiers- Nitrosomonas and Nitrobacter. Cooling tower biocides have many amines also present in them as well as zinc that can be toxic to the nitrifiers. Check your MSDS sheet on the chemicals used onsite.
Soluble Carbonaceous BOD > 25 mg/l in final effluent High pH (both Nitrosomonas and Nitrobacter are inhibited by un-ionized ammonia (NH3). Since Nitrosomonas is more sensitive than Nitrobacter, the result may be a high level of NO2- in the final effluent. Heavy Metals 3 mg/L to 23 mg/L -silver, mercury, nickel, chromium, copper, zinc Halogenated Solvents 0 mg/L Phenol and Cresol <20 mg/L Cyanides <20 mg/L
**Nitrite nitrogen concentrations greater than 20 mg/l can be inhibitory towards Nitrosomonas.
Salinity does not appear to inhibit the nitrification process in wastewater treatment plants.
Chlorides do not appear to be toxic unless very high. Typical chlorides concentrations were in the range 1000-2000mg/l. Tests with concentrations as high as 4000mg/l did not show a measurable inhibition of the nitrification process.
So what is a Total Nitrogen Balance?
Example from a customer site:
Final clarifier effluent- measure at least occasionally TKN and nitrites, daily measure ammonia and nitrates. The N not used by the carbonaceous bacteria as a nutrient source will only be converted by the nitrifiers and will still be in the system in one form or another as ammonia if not converted, or nitrate or nitrite if utilized by the nitrifiers.
OK, so the math does not add up. One thing people do not pay attention is the supernatant from the sludge digestor or belt press. Usually, when the carbonaceous bacteria go into endogenous respiration, they have started to run out of food. Some of the bacteria die off and re-release some of the nutrients. Two of the largest costs at a wastewater treatment plant are electricity and solids handling. Electricity can be high for mechanical pumps and aerators, especially if nitrifying.
It takes more time and electricity for mechanical energy for sludge digestion and nitrification processes. Balancing the cost of solids handling, vs. how much ammonia is returned from the supernatant can be critical. If the cost of solids handling is cheaper than electricity and nitrification, cut back a bit on your sludge digestion. Measure the ammonia present in the supernatant. If it is very high, you might be shooting yourself in the foot, (quite often seen at municipalities). The amount of savings on solids cost more in the long run to nitrify at a wastewater treatment plant.
Another thing where the math might not add up is if solids are held too long
in the clarifier and gassing or denitrification is occurring. When
If the nitrifiers run out of air in the clarifier, this can also inhibit
their growth, since many of the nitrifiers are returned to the front of the
system in the RAS. Watching your bed in the clarifier is critical to
nitrifiers.
Biomanagement Programs- Based on above all, a bioaugmentation program may be recommended depending on the need of the individual customer. The bioaugmentation program may consists of the addition of biological products supported by ongoing site services and laboratory analysis for monitoring and documenting the progress of the application at the wastewater treatment plant.
Nitrification Troubleshooting Newsletter Denitrification -Gassification Newsletter
For additional information on Biological products that can be used to help overcome Nitrification issues or to help the plant recover faster. MicroSolv 600L- Nitrifiers for bioaugmentation
The Critical 5 plus one- Alkalinity is critical for nitrification
Pollution Prevention Opportunities www.p2pays.org
|


 Temperature
Temperature
 What should be measured?
What should be measured?
